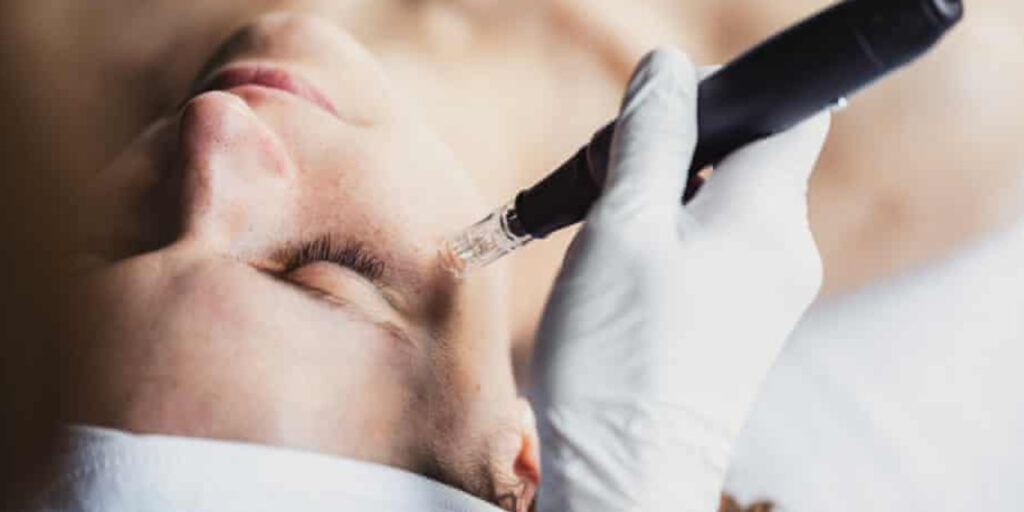
Experts have warned that banned exosomes derived from human cells are being used in UK beauty clinics, despite strict regulations prohibiting their use in cosmetics. The revelation has sparked alarm among scientists, who caution that such treatments could pose serious health risks, including potential virus contamination and disease transmission.
Human-Derived Exosomes Banned Under UK Law
Exosomes are microscopic membrane-bound particles naturally released by cells. In skincare, they are promoted for their rejuvenating properties and are used in high-end facial and microneedling treatments. While some formulations are derived from plants or fish cells, others are harvested from human sources such as umbilical cord blood or bone marrow—making them illegal under UK and EU cosmetic regulations.
The Department for Business and Trade confirmed: “Exosomes of human origin are banned in cosmetics that are sold in the UK.” Officials urge anyone concerned about the safety of beauty products to contact local trading standards or Citizens Advice.
Health Experts Sound the Alarm
Dr James Edgar, a University of Cambridge cell biologist, raised concerns after his wife was offered an exosome-based treatment in a hair salon. “You shouldn’t be having human cell-derived products and using them on other humans,” he said. “These are human biologics, and there’s a risk for disease transmission.”
Dr Guillaume van Niel, a leading researcher in the field of exosomes, highlighted another serious concern: the inability to reliably separate exosomes from viruses. “Viruses have the same size and physical properties as exosomes. If you isolate one, you get the other,” he explained. Van Niel emphasized that despite decades of research, scientists still don’t fully understand what exosomes are or how they work.
UK Clinics Caught Advertising Banned Treatments
An online investigation revealed that several UK cosmetic clinics had been advertising treatments involving human-derived exosomes. A clinic in Totnes, Devon, claimed its exosomes were “ethically harvested from consenting human donors,” while a London clinic promoted a microneedling product reportedly derived from umbilical cord stem cells.
When contacted, some clinics removed the promotional material and denied offering the treatments. Others claimed the descriptions were posted in error. Dev Patel, a former NHS doctor and cosmetic practitioner in Portsmouth, said he had unknowingly used banned exosomes before discovering their legal status. He now uses approved salmon-derived products and advocates for stricter industry regulation.
Call for Stronger Oversight in Cosmetic Industry
Patel expressed concern over the cosmetics sector’s lack of transparency and regulatory enforcement. “If anyone can set up a company and sell a product, how do you know you’re getting exosomes?” he said. He added that proper production of exosomes requires advanced technology, and warned that some products on the market may not be what they claim.
As the popularity of advanced cosmetic treatments grows, experts continue to call for tougher oversight and public awareness of what is being injected into consumers’ skin.